The excellent infographics on the top pharmaceutical sales compiled by the Njardarson group1 tell an interesting story from when they started in 2006 to today. In 2006, none of the top 10 or even the top 20 brand name drugs were biologics. Small molecules were king, with the first biologic, Amgen’s Enbrel, coming in at number 36. In 2021, things look rather different, with only 6 of the top 20 drugs being small molecules.
So where is this renaissance of small molecules, mentioned in the title? With biologics dominating headlines, and rightly so given the impact of the covid 19 vaccines in particular, one might be tempted to believe that small molecule drug discovery’s day is done, with fewer viable targets and drug space left to explore. However, this is far from the case.
Top 200 brand-name drugs by retail dollars in 2006

In 2021, 31 new drugs, accounting for 62% of new drug approvals were for small molecules , including new treatments for CNS disorders, viral diseases including HIV, different cancers, infections and heart and kidney diseases.
While market shift between biologics and small molecules is to be expected with technological advances on both sides of the fence, small molecules both alone and as part of mixed entities will continue to provide cost effective and reliable treatments to severe diseases.
https://njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster
Halford, B. Chemical and Engineering News. 2022, 100. New drug approvals held steady in 2021.
https://cen.acs.org/pharmaceuticals/New-drug-approvals-held-steady/100/i2
Ciulli, A.*, Farnaby, W.* Protein degradation for drug discovery. Drug Discov. Today Technol. 2019 Apr 31: 1-3
Schreiber, S. L.* The Rise of Molecular Glues Cell 2021 184 (1) 3-9.
Top 200 pharmaceuticals by retail sales in 2021
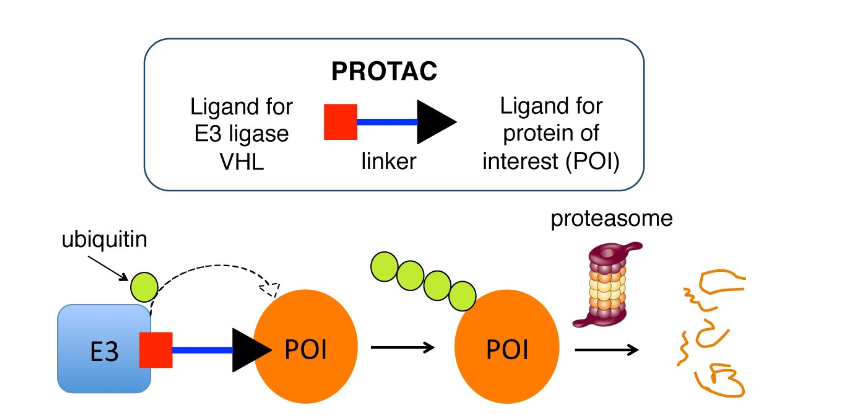
Newer and innovative approaches to small molecule research including new chemotypes and targeting approaches such as protein degradation, rather than inhibition or activation, have added additional dimensions into the small molecule field and are likely to feature in the drug approvals of the future.
PROTACs (PROteolysis TArgeting Chimera) consist of a target protein binder, a linker and an E3 ubiquitin ligase binder. To put it simply, one end of a PROTAC binds to the target protein that is implicated in disease (Protein of Interest (POI)) and is desirable to remove from the cell. The other end binds to an E3 ubiquitin ligase that can mark the target protein with a small protein called ubiquitin, this labels the protein as defective or damaged once attached to it. After that, the cell’s protein ‘shredder’ – the proteasome – recognises and degrades the labelled target protein.
Molecular glues4 can also be used for protein degradation. These are small molecules that do not consist of two protein targeting moieties attached by a linker such as a PROTAC, but instead are smaller single molecules which act as molecular adhesives by making two proteins stick together, which can have a degradation effect if one of the proteins is a ubiquitin ligase.
With two different types of small molecule being used for protein degradation, this field looks poised to feed into the pipeline of small molecule drugs going forward.
Sai has capabilities and expertise to address the challenges of PROTAC and molecular glue discovery and development. Our chemists are experiences in overcoming the challenges of synthesising PROTACS which have properties outside the traditional ‘Rule of 5’ space including diverse linker, E3 ligand and POI ligand types. Likewise, we have experience of adapting ADME assays to work with this type of modality. Our biology team have extensive capabilities and experience in validating degrader mechanism of action, E3 ligand ID, POI ligand ID (SPR and biochemical assays), degradation assys (HiBit and western blots) target engagement assays (NanoBRET and CETSA/NALSTA), ternary complex formation (SPR, FP/TR-Fret, NanoBRET and phenotypic and downsteam mechanistic (relevant downstream assays).
The future looks bright for new molecular entities from small molecule mediated protein degradation and Sai’s Discovery team are well placed to help clients address the challenges of discovering these new drugs, with our integrated biophysics, biochemistry, cell biology, in vivo pharmacology teams, alongside their colleagues in DMPK, computational, medicinal, and synthetic chemistry. Our development teams can then assist with the process research and scale up into the clinic and beyond. If you would like to explore the possibility of accessing Sai’s capabilities, please get in touch!
Author Name: Vicky Steadman, PhD, FRSC - Vice President Business Development and Integrated Partnerships
About Drug Discovery Innovation Programme 2022
Drug Discovery Innovation Programme is an invitation-only and one of the best platforms to learn the latest insights and develop lasting business relationships.
This year’s Drug Discovery Innovation Programme will highlight the challenges discovery pipelines have faced due to COVID-19 and will put a spotlight on thec adoption of technology to finding the solutions.
With over 100+ attendees, learn how modernization in R&D processes is fundamentally changing what the drug discovery research will look like in the next two to five years.
So, join us in 2022 for an in-person experience and 2-days of top-level strategic content and the current scientific insights, networking, and discussions from leading global pharmaceutical R&D executives.
Companies in attendance for 2022 will include Servier Pharmaceuticals, Monte Rosa Therapeutics, University of Oxford, WPD Pharmaceuticals, AISA Therapeutics, Anima Biotech, PDC*line Pharma, Eli Lillly and Company, Symphogen, IRB Barcelona, Axonis Therapeutics, Genentech, Arakis Therapeutics, Johnson & Johnson, Amgen, Revitale Pharma, Progenra Inc, CERo Therapeutics, Merck and much more.