Pharmaceutical artwork management is far from straightforward. From dosage instructions to safety warnings, every detail must be precise, up to date, and aligned with stringent global regulations. Frequent regulatory updates, multi-market variations, and complex approval workflows only add to the challenge, making accuracy and compliance increasingly difficult to maintain.
At ManageArtworks, we understand these challenges firsthand. With extensive experience working with pharmaceutical companies, we provide an end-to-end artwork management solution that ensures compliance, accelerates approvals, and minimizes errors. Our platform simplifies artwork creation, version control, regulatory approvals, and multilingual adaptations, helping companies streamline operations and reduce risks.
The ManageArtworks Solution
Unlike other industries, where packaging changes may be brand-driven, pharmaceutical artwork updates are often mandatory, driven by evolving regulations, new formulations, or safety updates. The process is highly complex, requiring a structured approach to prevent costly errors and ensure compliance. ManageArtworks is designed to address these challenges, providing pharmaceutical companies with a centralized and automated approach to artwork management.
-
End-to-End Artwork Workflow Management
Pharmaceutical artwork goes through multiple stages, from creation and regulatory approvals to market release and eventual obsolescence. ManageArtworks simplifies the entire workflow by integrating with regulatory and quality systems, ensuring smooth coordination at every step. When regulations change, the system automatically identifies affected artworks and routes them along with their Change Control numbers to the right teams for updates and approvals. By centralizing all artwork-related tasks, ManageArtworks helps companies stay compliant, reduce errors, and speed up time-to-market.
-
Regulatory Compliance and Data Security
ManageArtworks ensures full regulatory compliance with US FDA 21 CFR Part 11 and EU Annex 11 standards. All actions are securely logged, exportable, and backed by electronic signatures for approvals. Our system also meets stringent data security standards, including SOC certifications, ensuring the highest level of protection for sensitive pharmaceutical artwork data. Additionally, our team provides support for validation documentation (IQ/OQ) and assists with Performance Qualification (PQ) and Standard Operating Procedure (SOP) development, helping companies maintain compliance with industry regulations.
-
Centralized Pack Copy Management for Accuracy
Managing product packaging information involves multiple teams, each contributing critical details. ManageArtworks' Copy Manager acts as a single source of truth, ensuring all pack copy elements are centralized, standardized, and accessible. By streamlining content collation and approval workflows, teams can efficiently review and finalize information before it is incorporated into artwork, reducing the risk of inaccuracies and delays.
-
Ensuring Compliance and Version Control
Maintaining regulatory compliance is critical, and ManageArtworks offers robust version control to track all changes and approvals. With automated workflows, companies can ensure that all artwork updates meet regulatory requirements. The system can route regulatory changes to the appropriate teams, eliminating the risk of outdated information being used.
-
AI-Powered Proofing for Accuracy
Pharmaceutical packaging must be flawless, especially when handling multiple language versions. ManageArtworks incorporates AI-powered proofing tools to detect inconsistencies in text, graphics, and barcodes. By automating comparisons between artwork versions, the platform minimizes the need for manual proofreading, ensuring accuracy across all packaging materials.
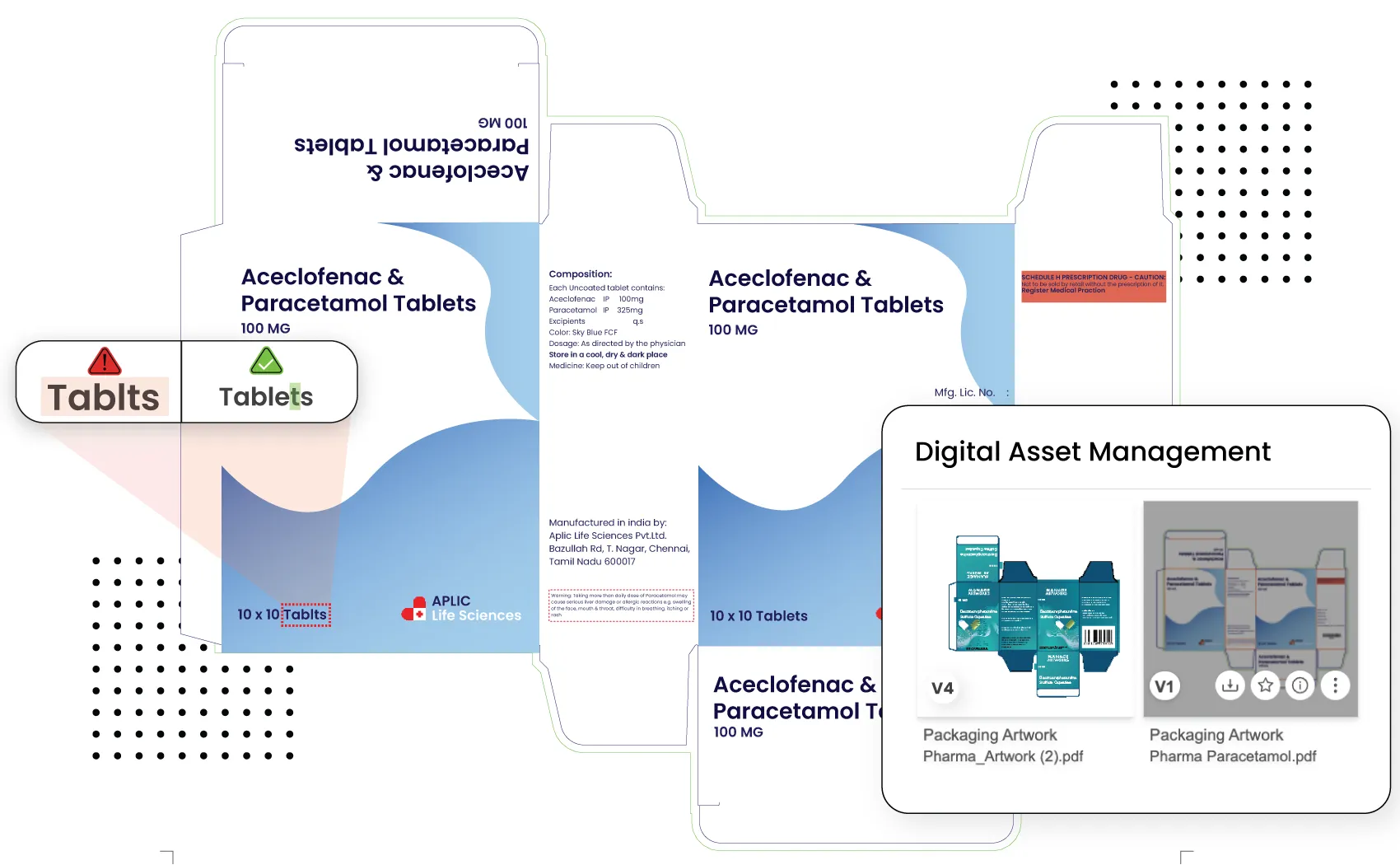
Secure Collaboration and Audit Trails
With multiple stakeholders like R&D, QA, regulatory, packaging, marketing, and legal teams involved in artwork creation, collaboration is essential. ManageArtworks provides a secure, cloud-based platform with user-based access controls, electronic signatures, and complete audit trails, ensuring transparency and accountability in every step of the artwork lifecycle.
Reducing Time-to-Market
Delays in packaging approvals can impact product launches and revenue. ManageArtworks streamlines the approval process by providing real-time tracking, automated notifications, and centralized storage. By eliminating bottlenecks, companies can bring their products to market faster while maintaining compliance and accuracy.
Future-Proof Your Pharmaceutical Artwork Management
The pharmaceutical industry operates under intense regulatory scrutiny, leaving no room for errors in packaging artwork. With evolving compliance requirements, multiple market adaptations, and complex approval cycles, managing artwork manually is not just inefficient, it’s risky.
With ManageArtworks, pharmaceutical companies can move beyond reactive fixes and embrace a proactive, digital-first approach to artwork management, ensuring precision, compliance, and efficiency at every stage.
Ready to streamline your artwork management? It’s time to work smarter with us. Book a demo now.
About Pharma Packaging & Labelling Forum 2025
Pharma Packaging & Labelling Forum 2025, organized by World BI, is an exclusive, invitation-only event that brings together industry leaders, regulatory experts, and solution providers to explore the latest advancements in pharmaceutical packaging and labelling. With a carefully curated agenda, the forum will deliver expert insights on Serialization & Track-and-Trace Technologies, Anti-Counterfeiting Measures, Regulatory Compliance & Evolving Standards and Sustainable & Eco-Friendly Packaging Solutions. Attendees will have the opportunity to network with top professionals from leading organizations, including Pfizer, Novartis, GSK, Roche, Merck, Sanofi, and more. Join us to gain valuable industry knowledge, exchange ideas with key stakeholders, and stay at the forefront of pharmaceutical packaging and labelling innovation.