Founded in 1965 with headquarters in Wilmington, MA and global offices, Analog Devices, Inc. (NASDAQ: ADI) is a global leader in sensors and technologies that bridge the physical and digital worlds to enable breakthroughs across many industries.
We combine analog, digital, software, hardware, and systems technologies into solutions that help drive advancements in digital healthcare, mobility, digitized factories, combatting climate change, and reliably connecting people with the world.
We have decades of experience in the healthcare industry as a key technology provider and partner for CT, MRI and digital X-ray systems, handheld ultrasound, vital signs monitoring, and much more. Our technology captures clinical-grade data and turns it into simple, trusted insights through our remote patient monitoring platform. By helping pharmaceutical partners accelerate drug development and medical device partners develop solutions to diagnose and treat patients better, we are helping to improve patient outcomes across the globe and making care possible in more places.
With revenue of $12.3 billion in FY23 and approximately 25,000 people globally working alongside 125,000 global customers, we ensure today’s innovators stay ahead of what’s possible.
Benefits of Partnering with Analog Devices
- Configurable solutions designed with patients in mind to drive compliance
- Tailored access to data (raw through insights) to help you understand the full picture
- Expertise and support in data management from collection through interpretation
- Trusted global supplier aligned with your quality, regulatory, and compliance policies
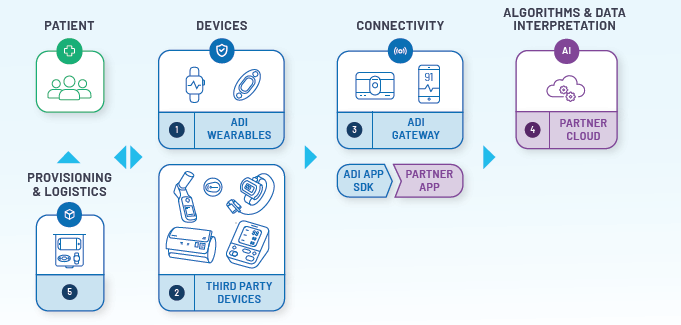
Elevate Your Remote Patient Monitoring Offerings
-
Universal Compatibility
Connects to weight scales, heart rate monitors, glucose meters, and more.
-
Customizable Settings
Enables the creation of custom mobile applications, providing partners with the ability to control and configure devices for a tailored experience (configure vital signs collected and sampling frequency).
-
Unified Device Integration
Enables access to multiple devices simultaneously through a singular, intuitive API.
-
3rd Party Device Integration
Seamlessly integrate time-synchronized data streams from multiple devices to create a clearer picture of patient health.
-
Cross-Platform
Supports both native Android and native iOS platforms, ensuring seamless functionality across diverse mobile ecosystems.
-
Future-Proof
Regular updates to our SDK ensure compatibility with the latest wearable and wireless medical devices, making the solution a future-proof choice for health monitoring.
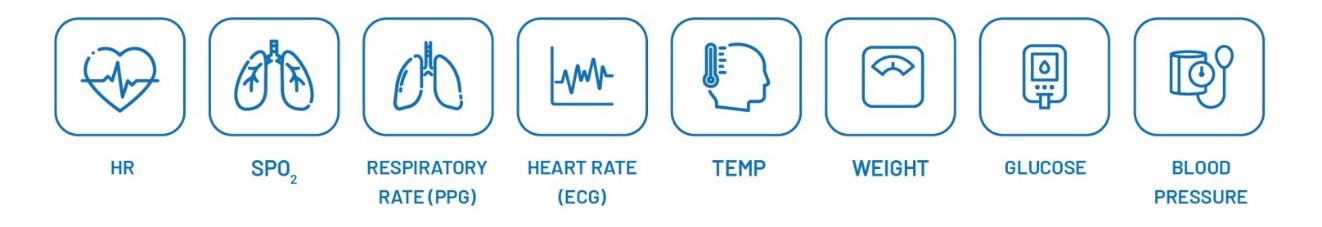
Parameters
About Clinical Trials Innovation Programme 2025
Clinical Trials Innovation Programme is an invitation-only, premium event bringing together a mix of clinical research professionals and service providers in one stimulating environment.
Join us face to face to learn from the best in the drug development industry and have an opportunity to learn from their experiences. With an agenda covering the future of Clinical trials and drug development post COVID, Clinical Trials Innovation Programme 2025 will feature tailored sessions presented by the leading experts from across the globe.
We are bringing together the best in the industry to help you learn and grow, and accelerate your drug development journey. The experts will include thought leaders and visionaries from Danish Medicines Agency, Bayer Pharmaceuticals, AstraZeneca The Janssen Pharmaceutical Companies of Johnson Johnson, Travere Therapeutics, Biogen, Vifor Pharma, Medical Products Agency and much more.